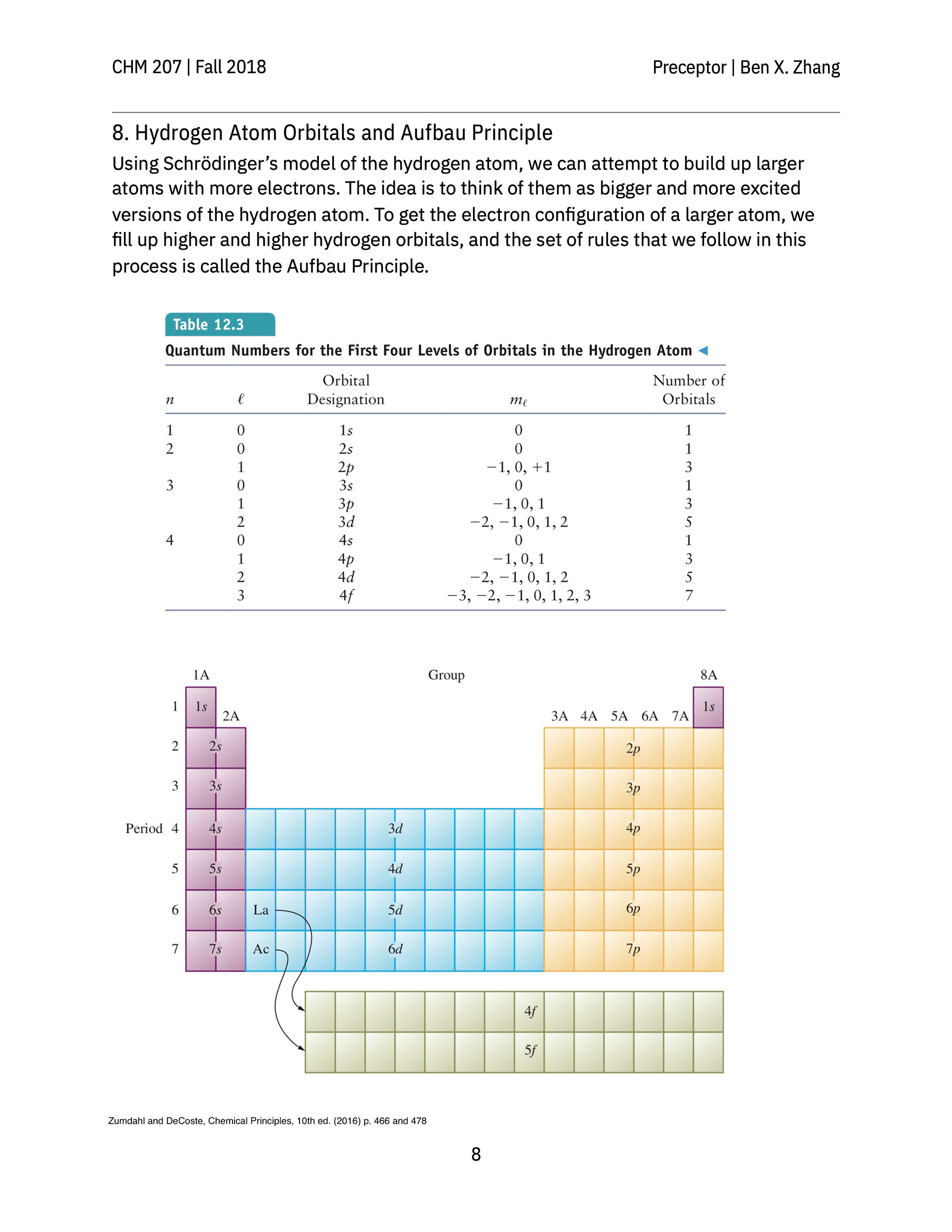
Presented here is a series of in-class hand-outs and after-class material designed for 10–15-person discussion groups, known at Princeton as precepts, that met once a week alongside the general chemistry lectures. The activities and concepts described on these pages aimed to help students appreciate intersecting perspectives on the nature of atoms and molecules as understood by working chemists today. Taken together, they are meant to equip students with a visual intuition on these invisible units of matter at the heart of modern chemistry.
> Energy levels of real atomic orbitals revealed by atomic emission lines compiled by NIST. In class, students are given the transition energy of a few strong emission lines for each element and have to match each pre-made "energy thread" to the correct element. When all energy threads are identified and arranged on the blackboard by their atomic number, interesting patterns emerge. This way of looking at atomic orbitals differed from the mental picture the students were familiar with when the aufbau principle was introduced. Contrary to the conceptual picture of filling progressively higher-energy orbitals of a ground-state atom, spectroscopists examine the neutral atom as if staring down an infinitely deep well. The highest occupied atomic orbital for each neutral atom lies on different energies depending on the particular quantum characteristics of the orbital as well as the nuclear charge.
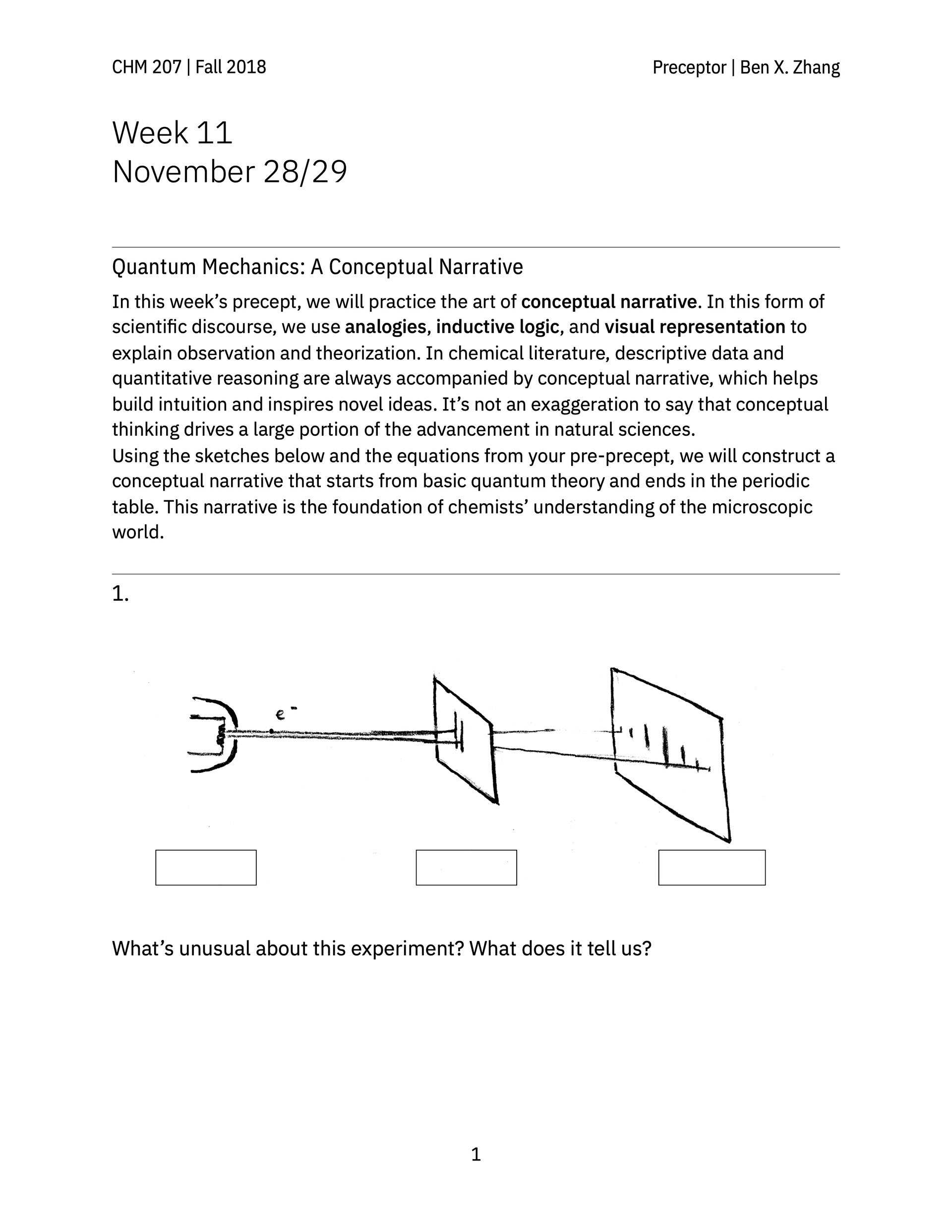
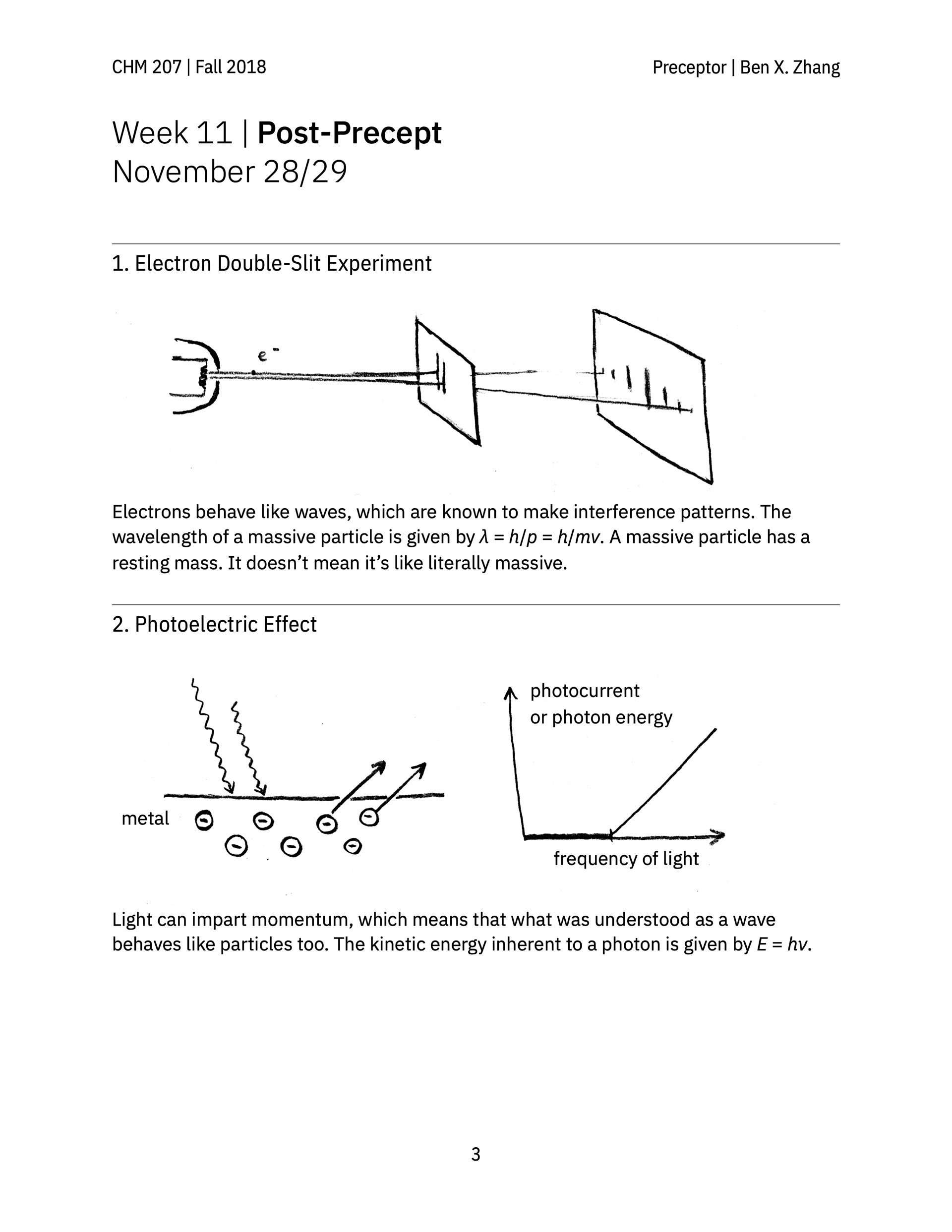
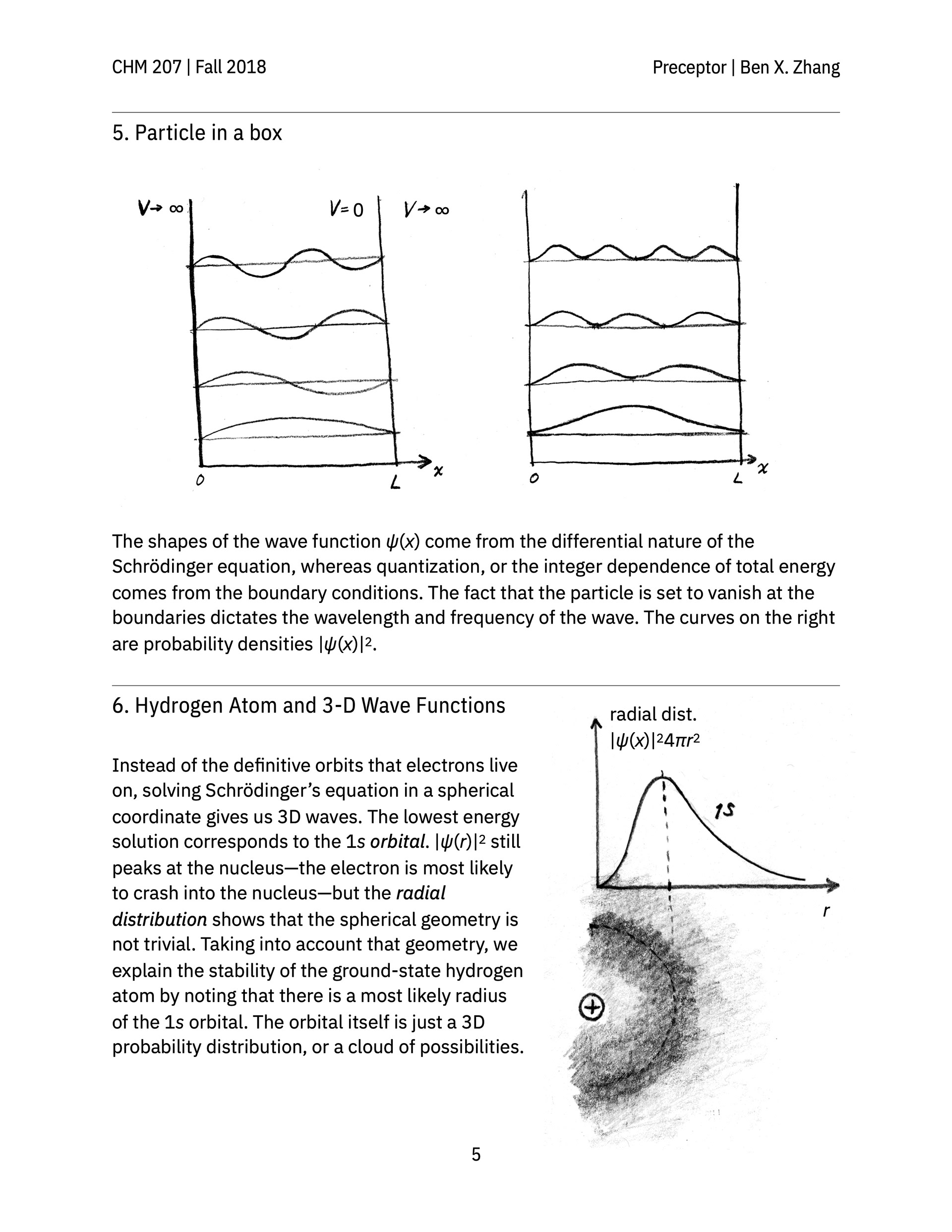
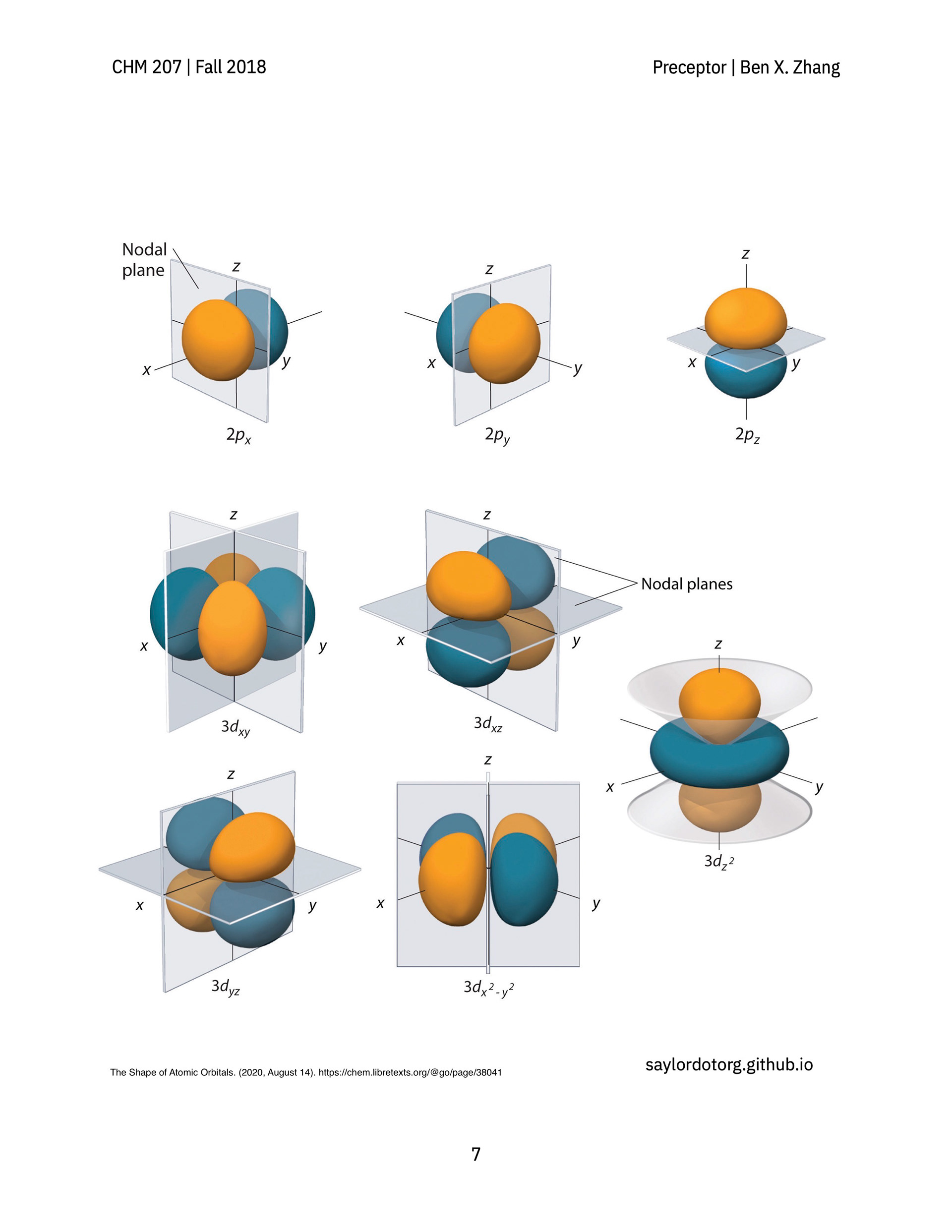
> A review guide that follows a conceptual narrative, going from the earliest experiments that revealed the wave-like nature of electrons all the way to atomic orbitals and the periodic table.


> A flowchart that summarizes the relationship between basic concepts concerning the nature of atoms and molecules taught in general chemistry.
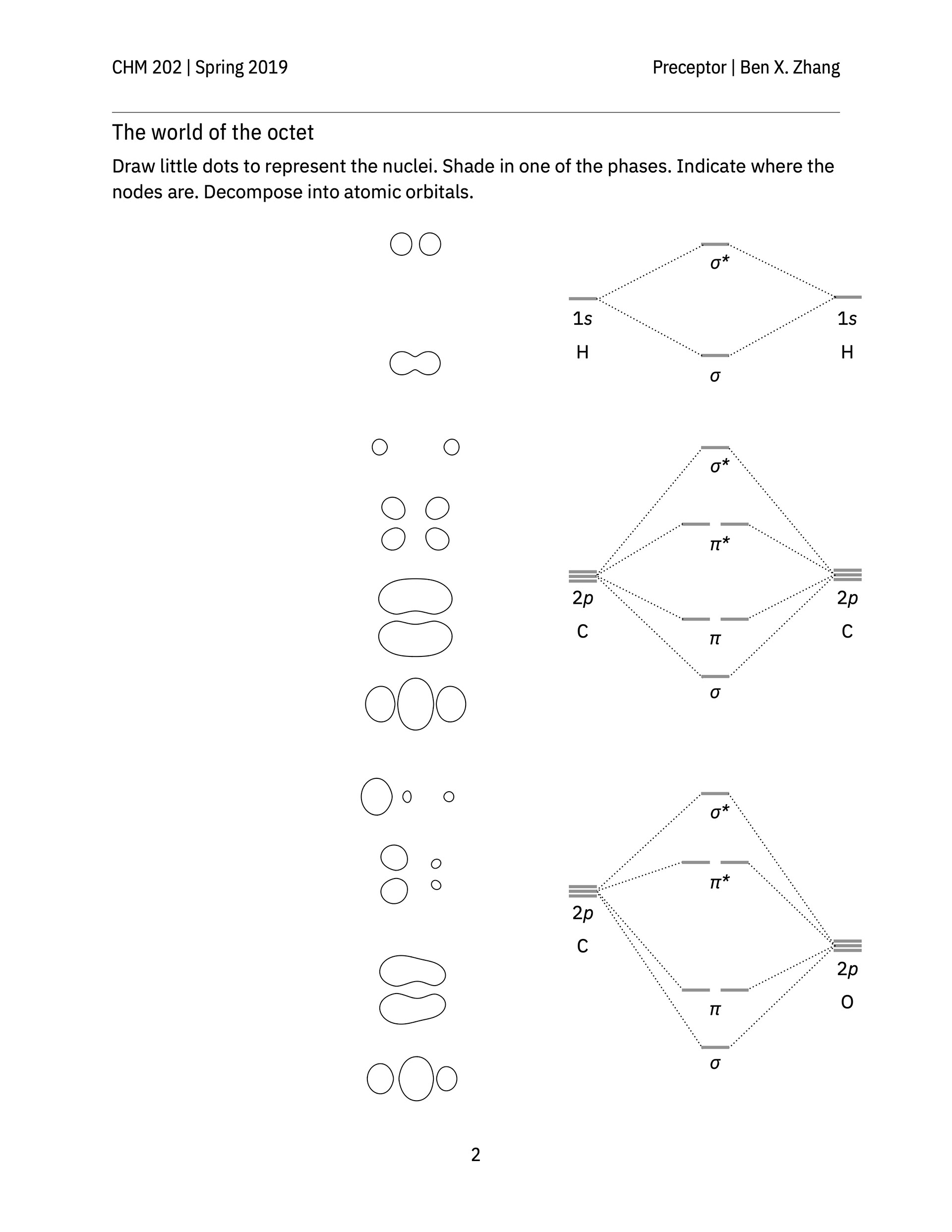
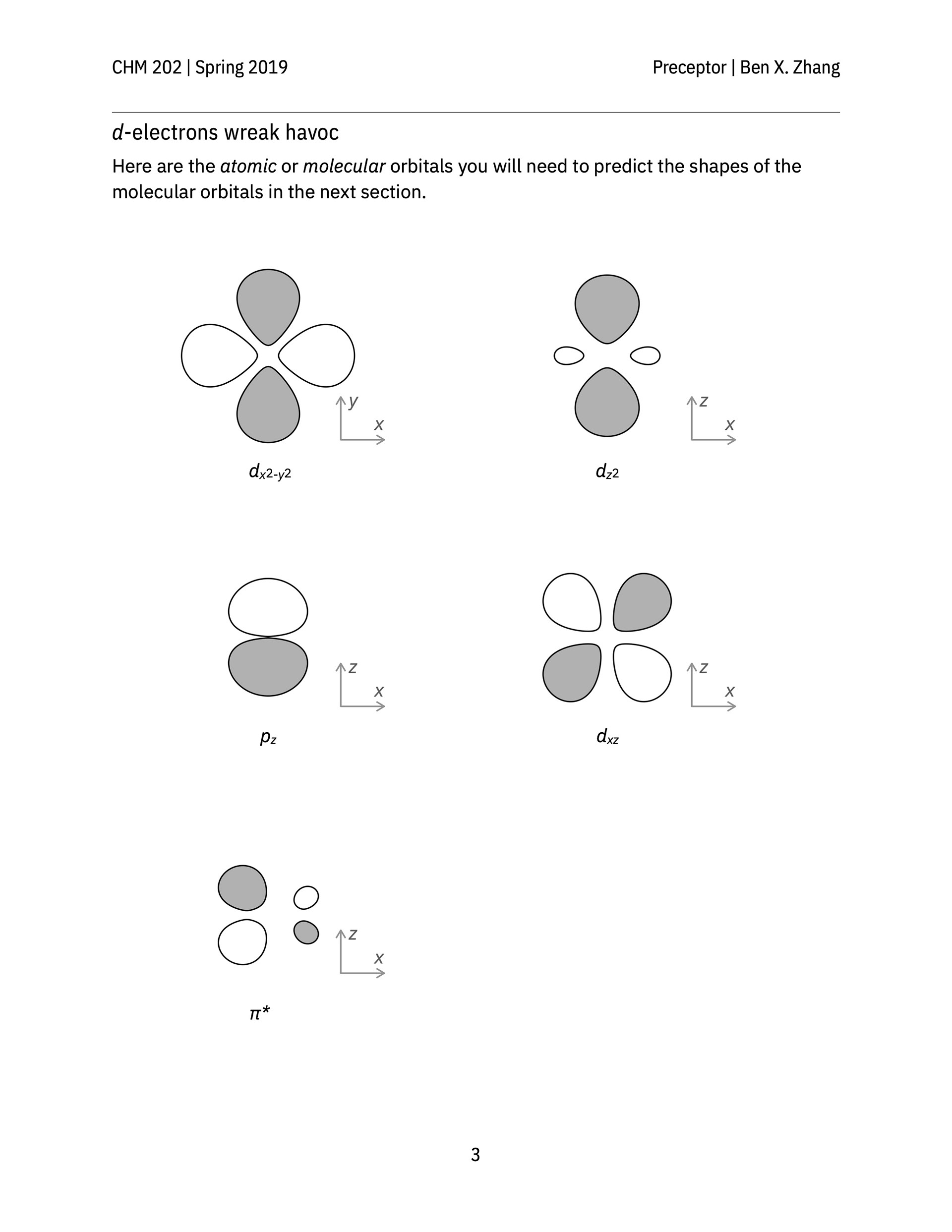
> A classroom activity that helps students see the shapes of bonding and anti-bonding molecular orbitals formed between s, p, and d-orbitals of constituent atoms.


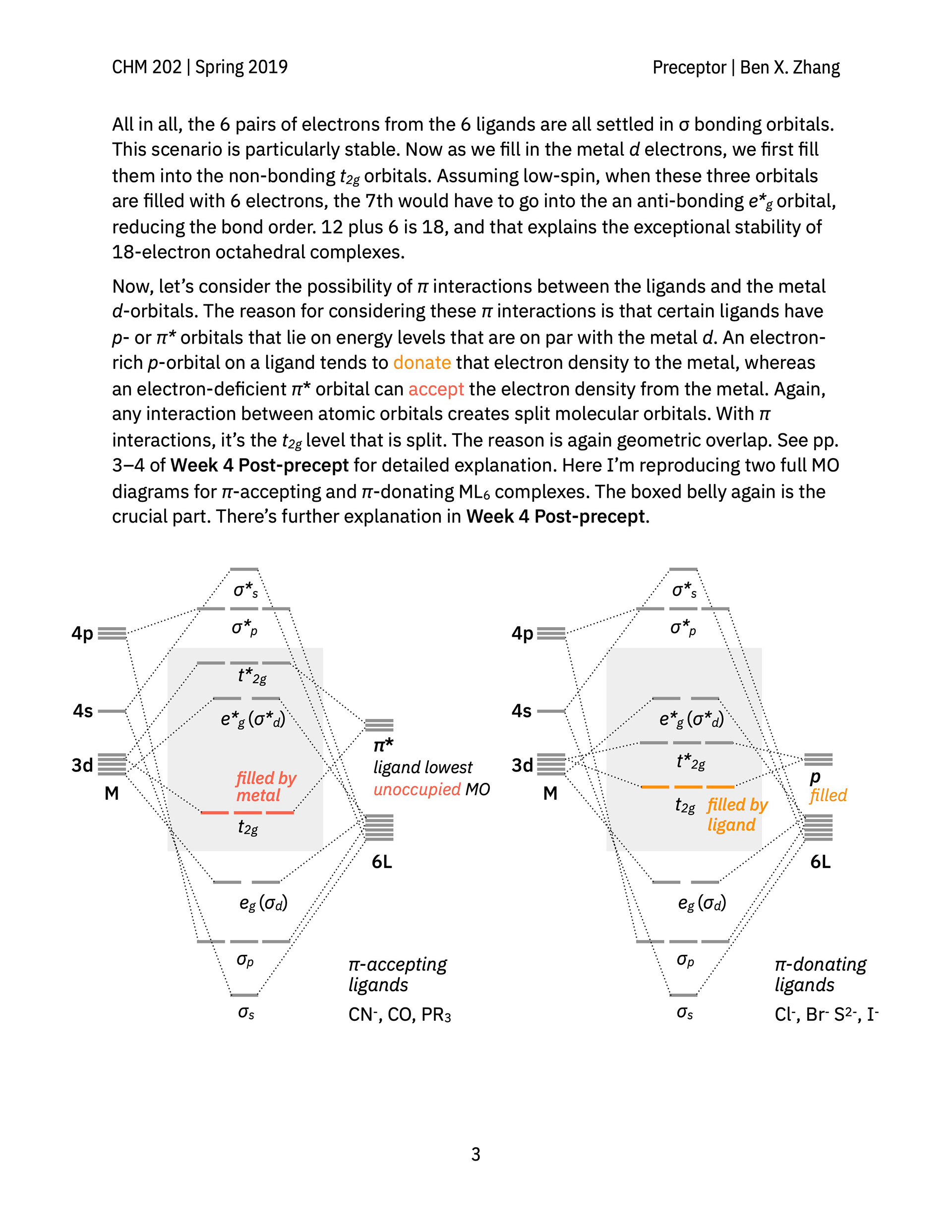
> A hand-out that explain the canonical molecular orbital diagrams of octahedral metal–ligand complexes with minimal pi interactions, pi-donating ligands, and pi-accepting ligands.

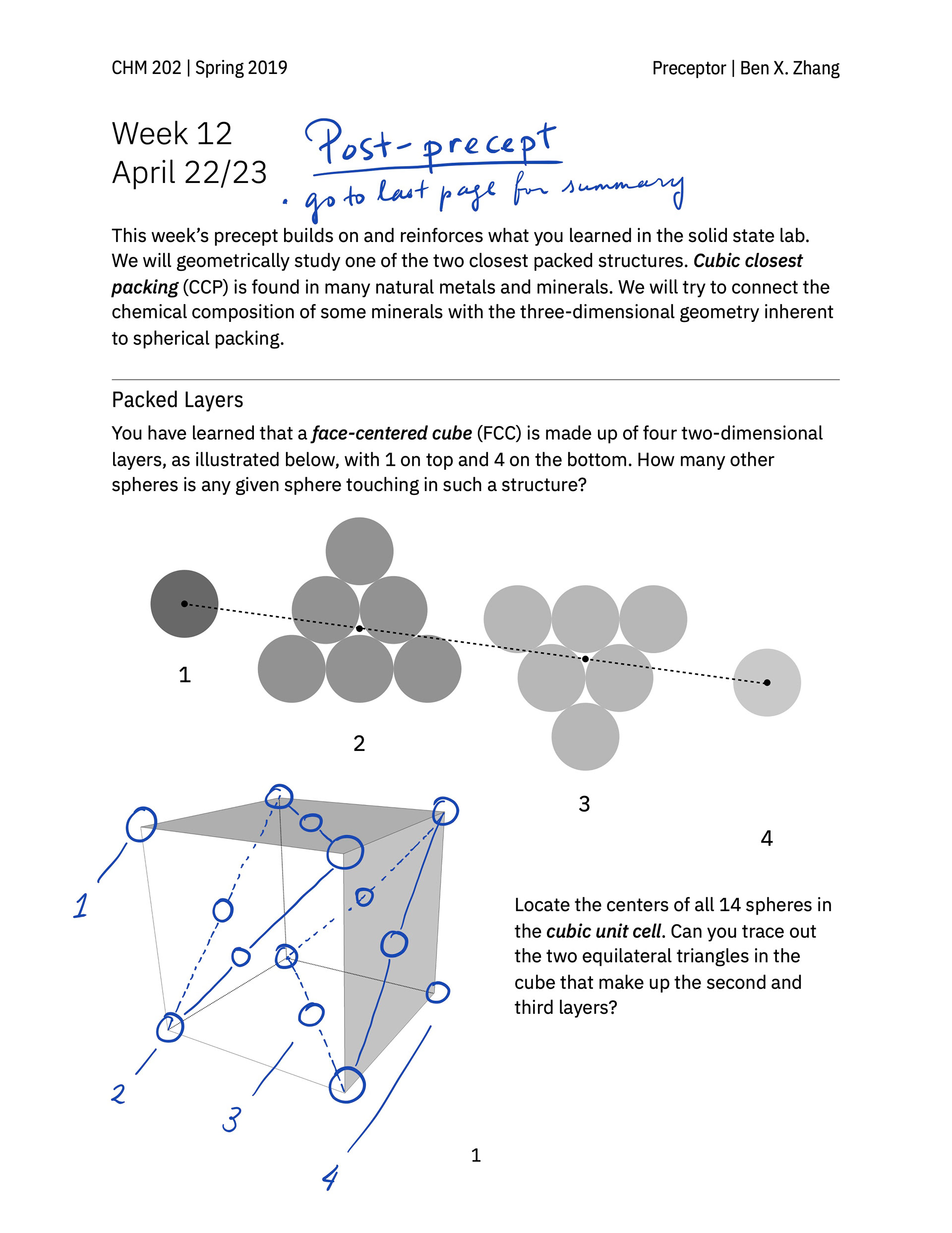
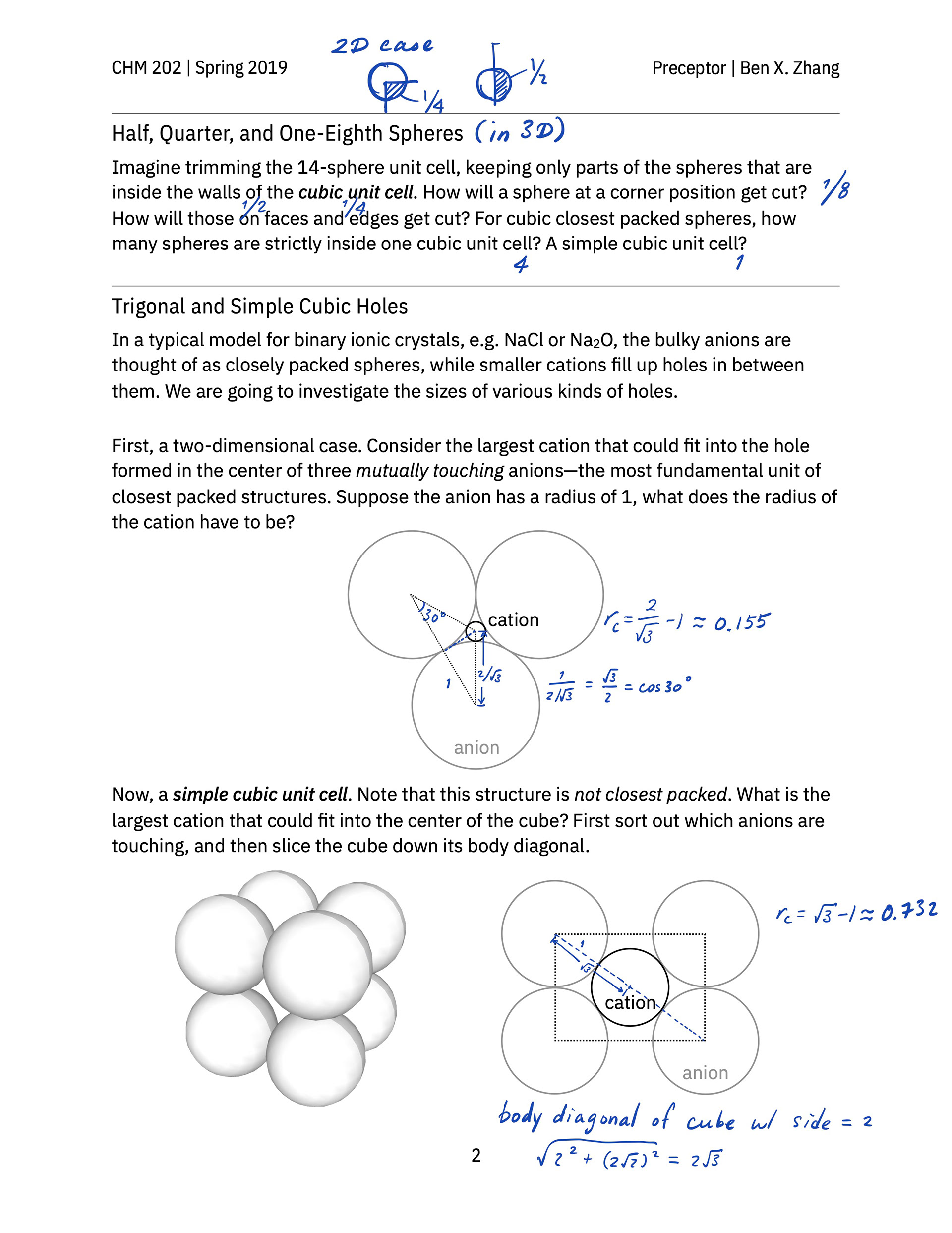
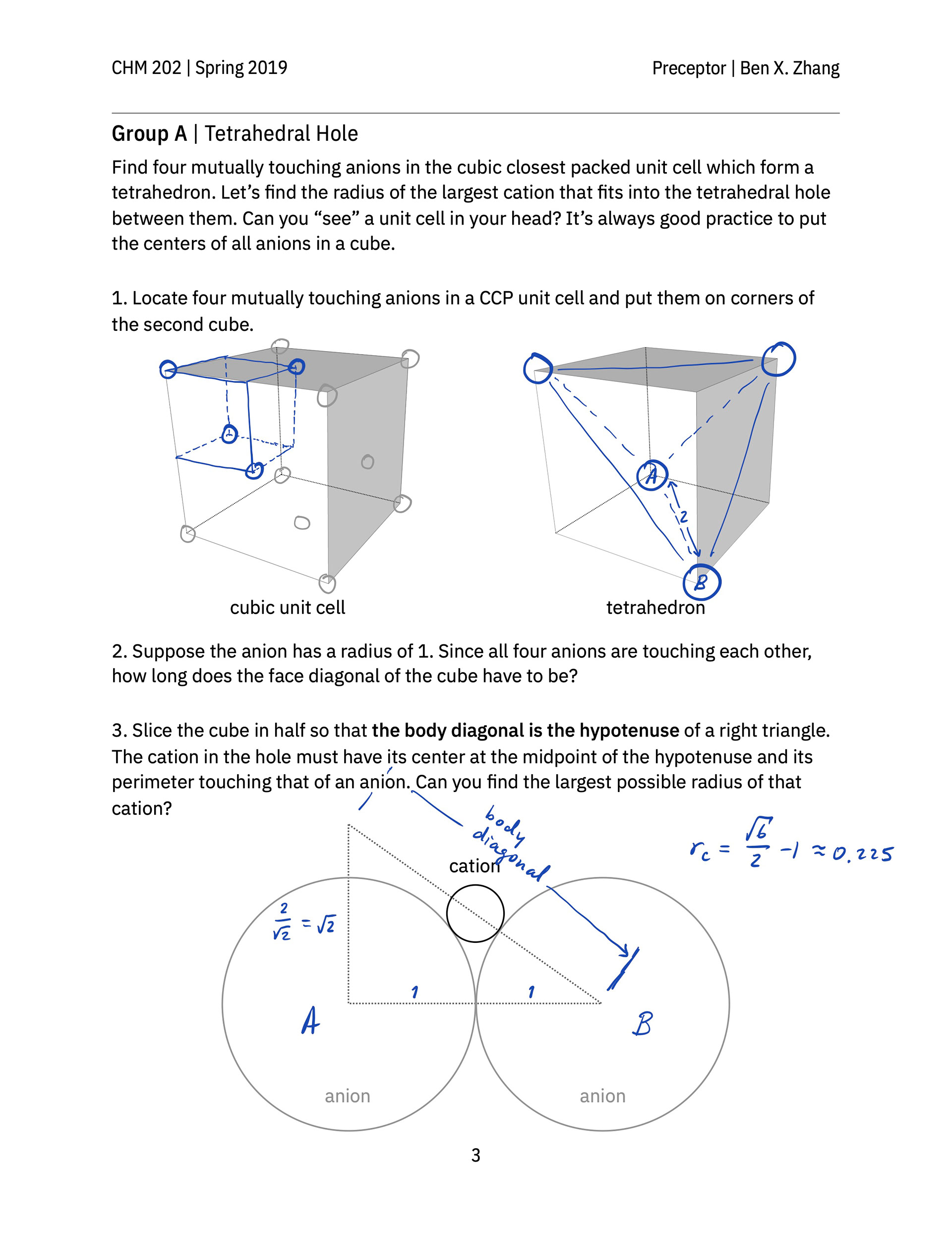
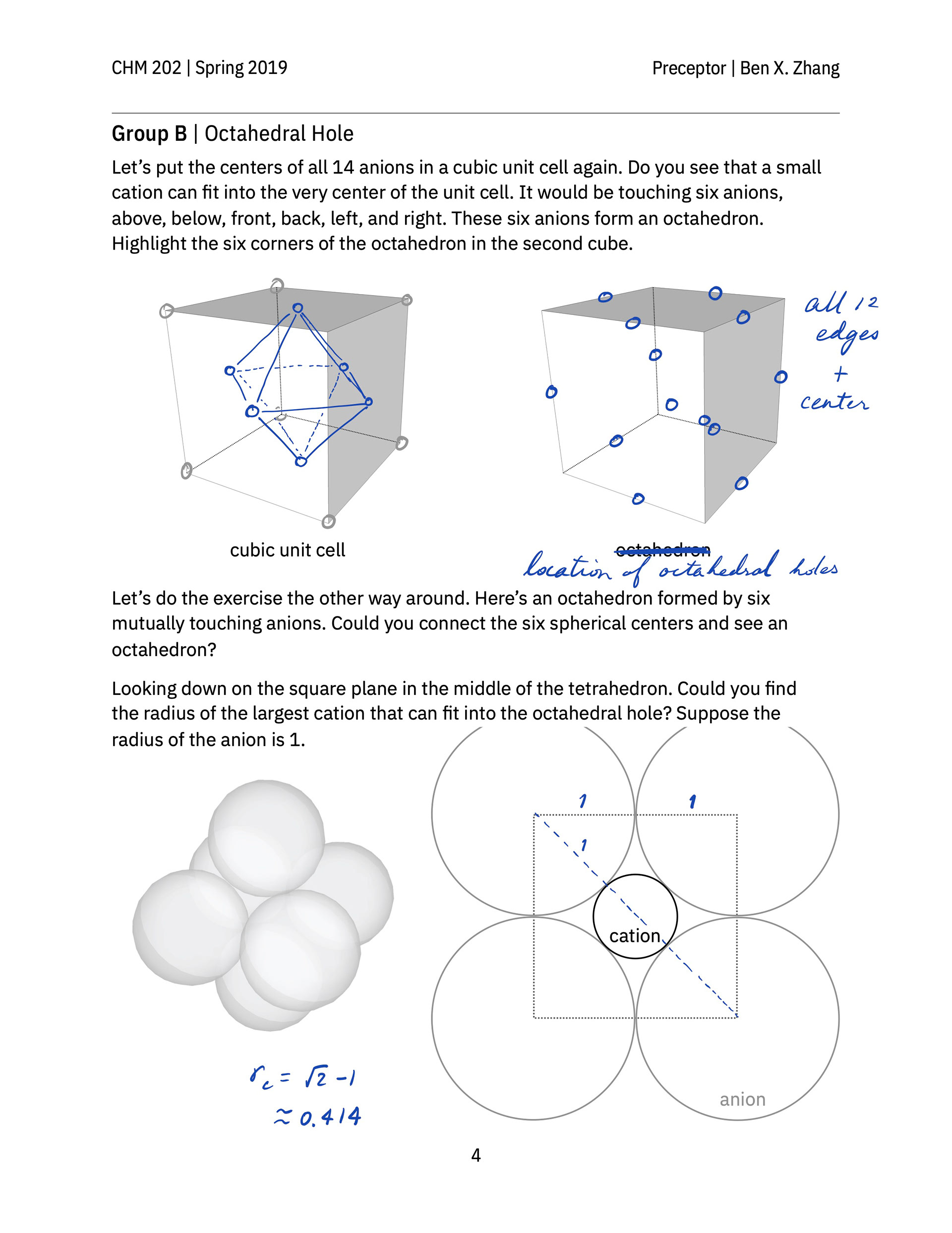
> A hand-out that guides students to build a face-centered cube lattice out of glass marbles in class and practice thinking about and sketching out the geometry of various kinds of holes in simple cubic and closest-packed cubic unit cells.