Here are in-class hand-outs intended to guide students through a group activity titled Titration on Paper. The activity is designed to familiarize students with typical problems encountered under the topic of acid–base titration in a standard general chemistry course. Students calculate by hand, or using an Excel spreadsheet, the pH of an acid–base mixture during putative titration experiments, between strong or weak acids and bases. Different groups were given different combinations of acids and bases.

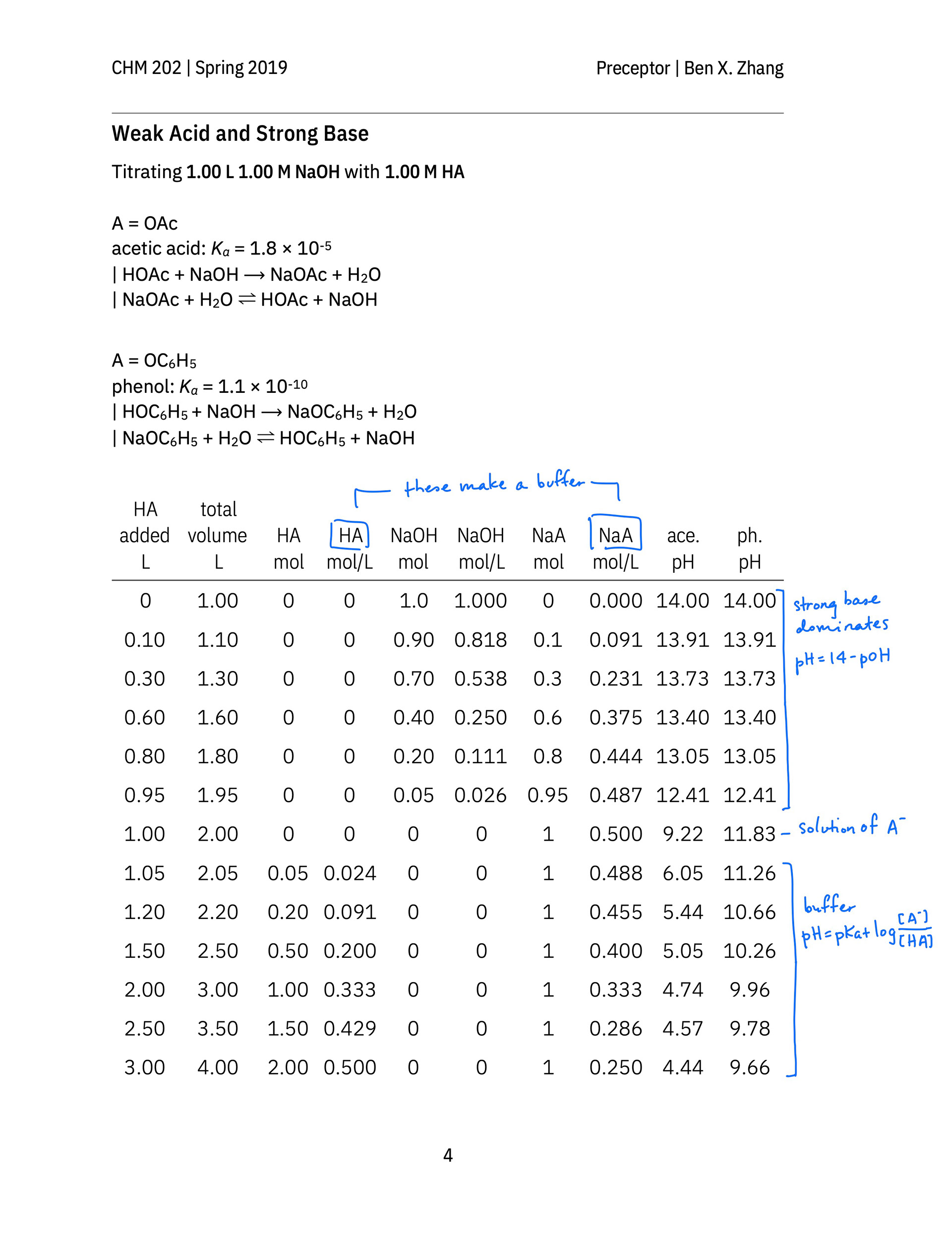
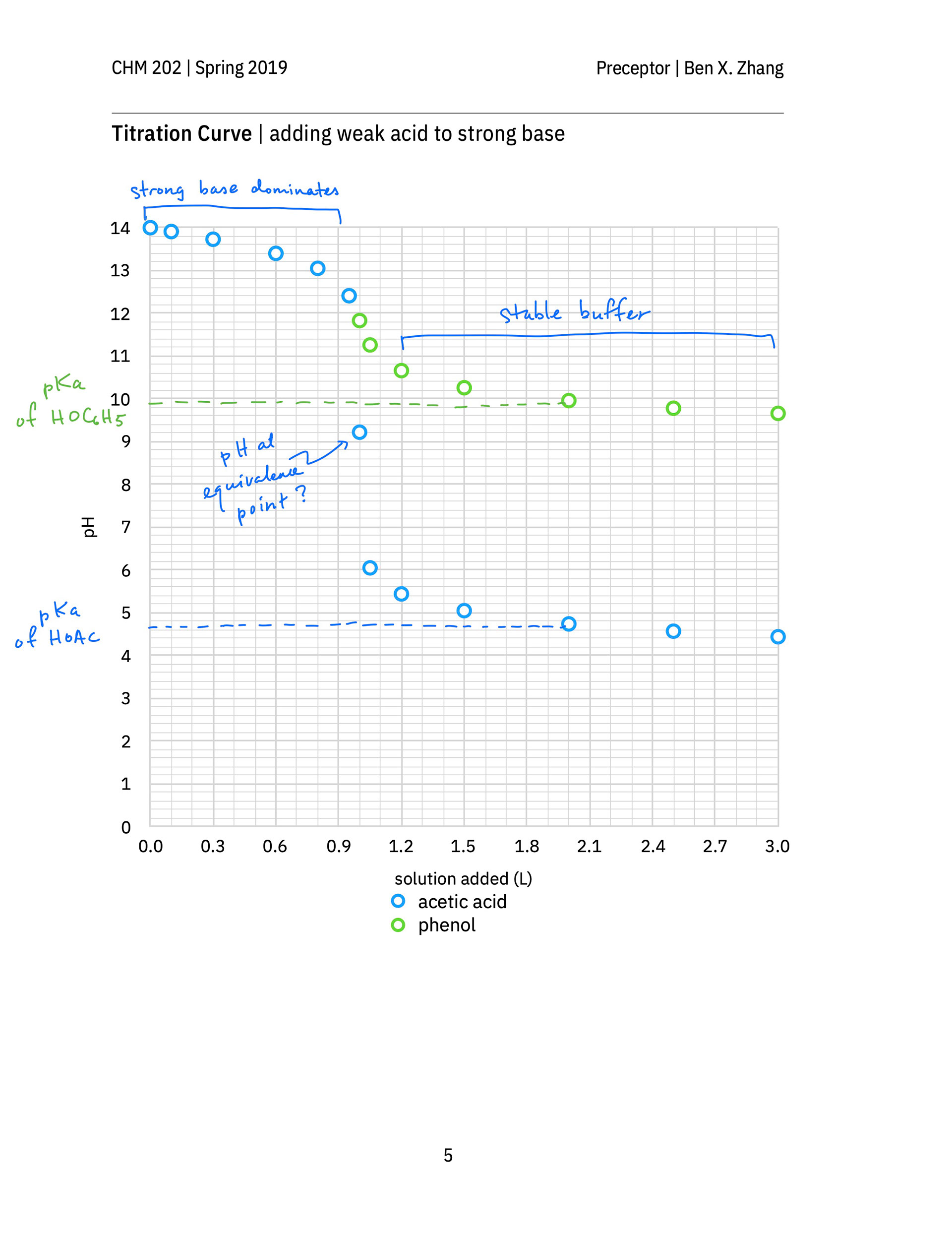
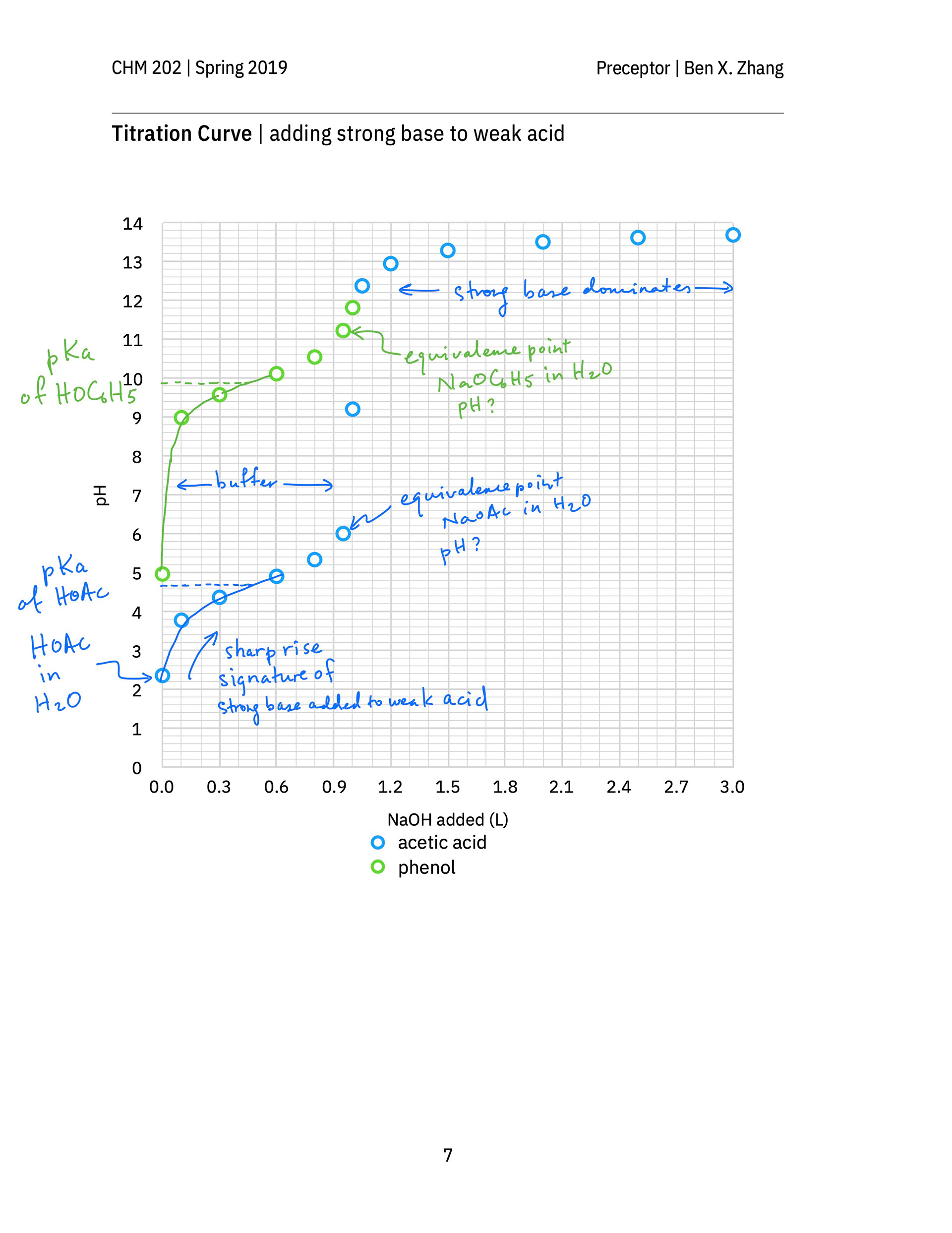
An annotated after-class hand-out not only contains solutions to the in-class exercises of all groups, but also clearly show students, in the context of a titration, when the Henderson–Hasselbalch equation should be used to calculate the pH of a weak acid or weak base solution. It also reminds students to review how to calculate the pH of a weak acid or base solution, which is what a strong–weak titration mixture becomes at the equivalence point. (The equivalence points on p. 7 of the hand-out are wrongly labeled. They should occur at 1.00 L solution added, not at 0.95 L solution added).